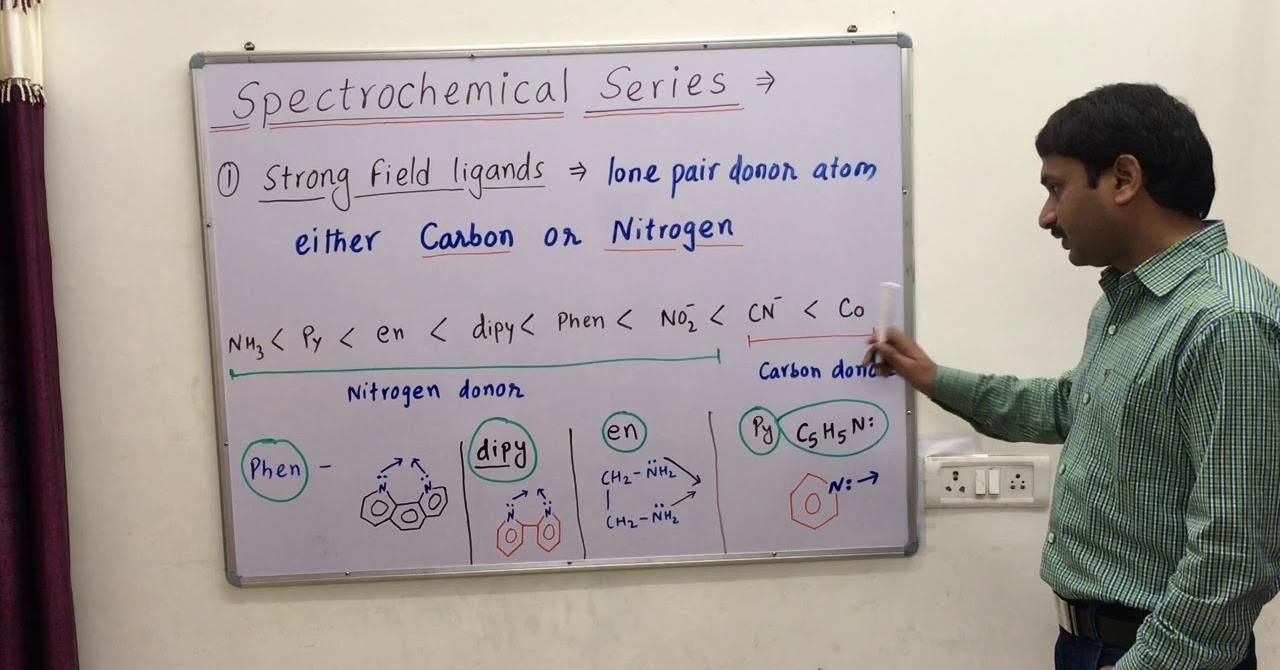
Are you curious to know what is spectrochemical series? You have come to the right place as I am going to tell you everything about spectrochemical series in a very simple explanation. Without further discussion let’s begin to know what is spectrochemical series?
In the fascinating realm of chemistry, the spectrochemical series stands as a critical tool, shedding light on the interactions between ligands and metal ions. In this detailed exploration, we’ll unravel the intricacies of the spectrochemical series, its formula, applications, and delve into real-world examples that showcase its significance.
What Is Spectrochemical Series?
The spectrochemical series is a classification system in coordination chemistry that ranks ligands based on their ability to influence the splitting of d-orbitals in metal ions. This series provides insights into the spectroscopic properties of coordination compounds, helping scientists understand the electronic structure and bonding in these complex entities.
What Is Spectrochemical Series In Chemistry?
In the realm of chemistry, the spectrochemical series serves as a fundamental concept within coordination chemistry. It specifically focuses on the impact ligands have on the electronic structure of metal ions, influencing the colors observed in coordination compounds.
What Is Spectrochemical Series Formula?
The spectrochemical series does not have a specific formula, but it is derived from experimental observations. The series is based on the ligands’ ability to cause electronic transitions in metal ions, resulting in different levels of d-orbital splitting. This splitting is crucial in determining the colors of coordination compounds.
What Is Spectrochemical Series Class 12?
For students in the 12th grade studying chemistry, understanding the spectrochemical series is essential. This concept plays a significant role in explaining the electronic structure and properties of coordination compounds. It is often a part of advanced chemistry courses that delve into the intricacies of transition metal chemistry.
What Is Spectrochemical Series: Explaining The Difference Between A Weak And Strong Ligand?
The spectrochemical series differentiates ligands into weak and strong categories based on their ability to cause d-orbital splitting. Strong ligands lead to significant splitting, resulting in fewer unpaired electrons, while weak ligands cause less splitting, leaving more unpaired electrons. This distinction has implications for the magnetic and spectroscopic properties of coordination compounds.
Spectrochemical Series Pdf
To delve deeper into the spectrochemical series, various resources, including PDF documents, provide comprehensive insights. These resources offer detailed explanations, charts, and examples that can aid students, researchers, and enthusiasts in grasping the nuances of this critical concept.
Application Of Spectrochemical Series
The spectrochemical series finds applications in various fields, including:
- Material Science: Understanding the electronic structure of coordination compounds aids in designing materials with specific properties.
- Bioinorganic Chemistry: It provides insights into the behavior of metal ions in biological systems, impacting fields like medicine and biology.
- Catalysis: The spectrochemical series influences the catalytic activity of metal complexes, playing a role in industrial processes.
You can collect more information on Getdailytech.
What Is Spectrochemical Series With Example?
Consider the example of the ligands CN− and H2O. CN− is a strong ligand that causes significant d-orbital splitting, resulting in a low-spin complex with fewer unpaired electrons. On the other hand, H2O is a weak ligand that induces less splitting, leading to a high-spin complex with more unpaired electrons. This example illustrates the practical application of the spectrochemical series in predicting the electronic structure of coordination compounds.
Conclusion
In conclusion, the spectrochemical series is a fundamental concept in coordination chemistry, providing a valuable framework for understanding the electronic structure and properties of coordination compounds. Whether you’re a student delving into advanced chemistry or a researcher exploring the applications of coordination complexes, the spectrochemical series remains a cornerstone in unraveling the mysteries of metal-ligand interactions.
FAQ
What Is Meant By Spectrochemical Series?
A spectrochemical series is the arrangement of common ligands in the increasing order of their crystal-field splitting energy (CFSE) values. The ligands present on the R.H.S of the series are strong field ligands while that on the L.H.S are weak field ligands.
What Is Strong And Weak Field Ligand?
Ligands that produce a large splitting are called strong field ligands, and those that produce a small splitting are called weak field ligands. An abbreviated spectrochemical series is: Weak field I- < Br- < Cl- < NO3- < F- < OH- < H2O < Pyridine < NH3 < NO2- < CN- < CO Strong field.
What Is The Order Of The Spectrochemical Series?
Spectrochemical series is given below: I-<Br- <S2- <Cl-<OH- <C2O42- <H2O <NCS-<Py <NH3 <en <NO2+ <CN-<CO.
What Is The Difference Between Sfl And Wfl?
Weak field ligands contain atoms from both the lowest energy ground state and highest energy excited state, while strong field ligands contain atoms in the higher energy state. Weak field ligands are stronger in terms of their ability to form intermolecular interactions than their strong field counterparts.
I Have Covered All The Following Queries And Topics In The Above Article
What Is Spectrochemical Series With Example
What Is Spectrochemical Series In Chemistry
What Is Spectrochemical Series Formula
What Is Spectrochemical Series Class 12
What Is Spectrochemical Series Explain The Difference Between A Week
Spectrochemical Series Pdf
Application Of Spectrochemical Series
What Is Spectrochemical Series